In 2013, we expect 232,340 new invasive breast cancers plus 64,640 in situ (pre-invasive) cancers, and 40,030 deaths; 2240 of those cancers will be in men with 410 deaths. We also expect 22,240 ovarian cancers to be diagnosed this year, and 14,030 deaths.
Only 5-10% of breast cancers and 14% of ovarian cancers occur from a genetic predisposition, which means a mutation inherited from either parent allows cancer to grow uncontrolled. The most common genetic mutation for which we can test is called, BRCA (pronounced, brack’-uh), and was discovered in the early 1990s.
 We’ve been working hard on this. Add your e-mail address and we will tell you as soon as it launches.
We’ve been working hard on this. Add your e-mail address and we will tell you as soon as it launches.
BRCA Mutation Quick Facts
- BRCA stands for BReast CAncer. Normal BRCA genes help you fight cancer when it happens in your body. Some families carry mutated or broken BRCA genes that can be passed down from one generation to the next.
- Women carrying a BRCA mutation have up to an 87% lifetime chance of breast and 54% chance of ovarian cancers (vs. general population: 12% breast and < 1% ovarian).
- While these risks seem staggering (and they are!), a woman’s risk is never that high on any given day in her life. The following table (from King, et.al.) stratifies the risks of getting breast and ovarian cancer by decade of life, and compares those risks to the general population for breast cancer:
Risk Gen Popn BRCA1 BRCA1 BRCA2 BRCA2
by age (breast) (breast) (ovarian) (breast) (ovarian)
30 0.06% 3% 0.00 0.06% 0.00
40 0.46% 21% 3% 17% 2%
50 1.86% 39% 21% 34% 2%
60 4.26% 58% 40% 48% 6%
70 7.66% 69% 46% 74% 12%
80 11.36% 81% 54% 85% 23%
- Men can inherit this mutation, and their breast cancer risk is up to 8% (vs. 1 in 1000 general population), with a 20% chance of prostate cancer (vs. 14% general population).
- BRCA-2 is associated with other cancer risks: prostate, melanoma, and pancreatic. (To date, increased colorectal cancer risk has not been established, but is suggested by some studies.)
- In those BRCA-1 carriers who get breast cancer, 85% will have a more aggressive “triple negative” subtype (vs. 15% general population).
- Only 5-10% of breast cancers and 14% of ovarian cancers occur from a genetic predisposition – the rest do not.
- General population risk for a BRCA mutation is 1 in 500 people, but those of Ashkenazi Jewish heritage are 1 in 40
- An estimated 19 million people in the U.S. have family histories of breast and ovarian cancer that meet the US Preventive Services Task Force criteria for hereditary cancer testing; that means they have a > 5-10% chance of carrying the gene mutation. As of this year, only 1% have tested. If all 19 million tested, around 1 million would be found to carry the BRCA gene mutation.
- Testing is done by an oral rinse or simple blood draw and can be done in the convenience of your own home with the Color Hereditary Cancer Test.
Get discounted Tier 2 tickets while they last. Watch the Summit via on-demand video from anywhere or attend in person at the breathtaking oceanfront Terranea Resort in Southern California.
8 Red Flags that Indicate a Possible BRCA Mutation
To assess your personal risk for a BRCA mutation, take this simple Genetics Quiz
1st, 2nd, or 3rd degree relatives – from maternal or paternal sides – with breast cancer before age 50 or ovarian cancer at any age;
Ashkenazi Jewish heritage (Ashkenazis are Eastern European Jewish from Germany, Poland, Lithuania, Ukraine and Russia, as opposed to the Sephardic Jewish population primarily from Spain, parts of France, Italy, and North Africa);
Any male relative with breast cancer;
Any relative who is a known BRCA mutation carrier (the child of a carrier has a 50% chance of inheriting the mutation);
Breast cancer in self prior to age 50;
Two breast cancers in self, any age;
“Triple negative” breast cancer in self;
Two or more family members with breast, ovarian, pancreas, prostate, melanoma, uterine, colon, and stomach cancers (this flag also captures possible non-BRCA inherited genetic mutations associated with breast or ovarian cancer).
Follow Power Up
Follow Power Up
Insurance and Privacy Concerns
- The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) made it illegal in the United States for group health plans to consider genetic information a “pre-existing condition” or to use it to deny or limit coverage. HIPAA imposes federal penalties for breaches of medical confidentiality.
- The Genetic Information Nondiscrimination Act (“GINA”) of 2008 protects people in both group and individual health plans from genetic discrimination. It makes it illegal for employers to use genetic information in the hiring, firing, job assignments, and promotions of their employees. GINA also prohibits health insurance companies and employers from requesting or requiring an individual to take a genetic test.
- To date, no evidence of life insurance discrimination following BRCA1 and BRCA2 testing has been found.
Management Options For BRCA Carriers
PLBC’S APPROACH: When first meeting a woman newly diagnosed with a BRCA mutation, our immediate goal is to learn as much about her preconceptions and personality as possible. How has the cancer experience of affected relatives or friends colored her perception of treatments and side effects? Is she heterosexual, lesbian, or both? Is she in a stable relationship, married, or single? Has she completed childbearing? If no, does she desire children? Is breastfeeding a strong priority? Do we need to discuss freezing eggs or embryos if she is between 30-40? How much do her breasts “mean to her” in terms of body image, sense of sexuality, sexual pleasure, and defining her femininity. Which at-risk organ creates more anxiety for her: breasts or ovaries, and why?
Two main strategies exist for BRCA carriers: surveillance and surgery.
Surveillance involves a combination of imaging and exams, as well as possibly taking risk-reducing medications.
Surgical interventions require removing the organs as risk, namely, the breasts and ovaries.
- Breast & Ovarian Surveillance
PLBC’S BRCA plan for women: Beginning at age 18, or 10 years younger than the youngest relative with breast cancer, every 3 months, you have breast imaging or an exam. An example plan follows:
- Month 1 = Mammogram (wait until 30 years old); Whole breast screening ultrasound (begin at 18).
- Month 4 = Clinical breast exam with a breast specialist.
- Month 7 = Breast MRI, timed to menstrual cycle days 7-10 (begin at age 25; day 1 is the day bleeding starts); Contrast Enhanced Spectral Mammography CESM for those who cannot have an MRI.
- Month 10 = Clinical breast exam with a breast specialist.
- Every month = Self breast exams (cycle days 7-10). If you don’t know how to do a self breast exam, please watch our how-to video.
For those who prefer twice-a-year surveillance, we combine the imaging with clinical breast exams, and meet every 6 months.
Ovarian surveillance begins at age 30, or 10 years prior to youngest relative with ovarian cancer, and includes the following:
- Transvaginal Pelvic ultrasound every 6 months (cycle days 1-10)
- CA-125 blood marker testing every 6 months (after cycle day 5)
- Pelvic exam by gynecologist every 6 months
PLBC’S BRCA-2 Additions:
- At 35, get an annual MRI of the pancreas, along with blood levels for liver function and pancreatic enzymes.
- At 25, begin annual skin and eye exams for melanoma.
PLBC’S BRCA plan for men:
- At 35, clinical breast exam annually
- At 35, self breast exams monthly
- At 40, baseline mammogram
- At 40, prostate and yearly PSA checks.
- BRCA-2 additions as above.
- Risk Reduction Medications AKA Chemoprevention
- Oral contraceptives (birth control pills)
- Reduce the risk of ovarian cancer up to 60%. Reduction increases with duration of use.
- Do not seem to increase breast cancer risks in BRCA1/2, but some studies suggest a breast cancer increase with OCPs used in women prior to first pregnancy.
- Tamoxifen
- An anti-estrogen pill that blocks estrogen receptors throughout the body
- Once diagnosed with breast cancer, Tamoxifen decreases breast cancer in the opposite breast in both BRCA-1/2 by 50%
- Reduces first time breast cancer risk in BRCA-2 (not BRCA-1) by up to 62% (NSABP-P1 study)
- Side effects – menopause symptoms: hot flashes, night sweats, decreased libido, vaginal dryness, mood swings (see Menopause Miracle to counteract these symptoms)
- Complications – blood clots, stroke, uterine cancer
- Prophylactic Operations
- Prophylactic (“Preventive”) Bilateral (“Double”) Mastectomy
- Definition of “Prophylactic”: Acting to defend against or prevent something, especially disease; protective.
- Prophylactic mastectomy reduces future breast cancer risk to less than 10%, but with a truly excellent mastectomy technique, the risk should be under 2%, even when keeping the nipples.
- What is the ideal age? 10 years younger than the youngest affected relative with breast cancer; or between ages 30-40; or earlier per patient desire. Why the 10 years advice? Scientific studies show that the doubling rate of a cancer cell is such that the first cell most likely mutated 5-8 years before the cancer could ever be detected and diagnosed. So, the 10 years is meant to stop cancer before it starts.
- New Technique #1: Gone are the days of a “slash” across the entire chest, or an unrealistic “grapefruit” attached to the chest, meant to resemble the sloping beautiful breast that was once in its place. Minimally visible incisions using Nipple-sparing, Areolar-sparing, and Skin-sparing choices, as well as diverse oncoplastic techniques available today can leave a woman feeling sexy and strong inside her own skin.
- New Technique #2: A Nipple Delay procedure should be considered whenever the nipples are thought to be at high risk for either disease or insufficient blood supply after a planned nipple-sparing mastectomy. The delay, performed 1-2 weeks prior to the actual mastectomies, uses the planned mastectomy incision and lifts half of the skin off of the breast surface. Also, a small disc of the tissue directly behind the nipple and areola is removed and analyzed by a pathologist. This procedure rules out disease in the breast ducts directly behind the nipples, which would make nipple preservation dangerous. Additionally, a nipple delay recruits extra blood flow to the nipple and skin, lessening the chances of nipple and skin loss due to insufficient blood supply after mastectomy. Since starting this technique in 2008, my loss of skin and nipple after mastectomy has decreased to < 2%.
- New Technique #3: Whenever a breast contains cancer and the armpit lymph nodes cannot be felt on exam, we routinely perform a sentinel node biopsy, which is removal of the first nodes receiving the breast lymphatic drainage – in this way, we find out if cancer spread beyond the breast. The chances of finding cancer in a breast removed prophylactically range from 2-8%. Until now, a woman undergoing prophylactic mastectomy had two choices: (1) take the sentinel node(s) in case cancer is found in her breast, or (2) don’t take the node(s). This dilemma has been resolved with my new technique, called Prophylactic Breast Dye Injection. PBDI allows the node to be identified, but not removed, giving more control and peace of mind to women.
- Reconstruction options vary depending on your desired aesthetic outcome, body habitus, general health, prior breast operations, and radiation exposures. The two broad categories of reconstruction include implants and flaps.
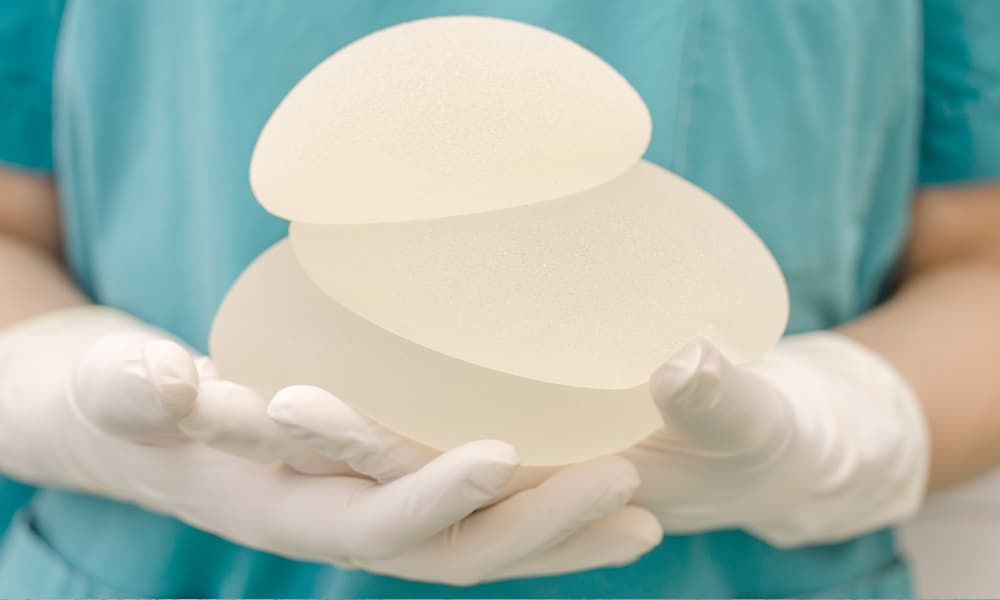
- Implants are the most common reconstruction, often requiring two stages, whereby a tissue expander is placed prior to the final implant. A tissue expander is a deflated implant that goes behind the pectoral muscles and gets slowly inflated with saline over a period of 2-3 months, until the desired volume is reached. A second operation is performed to swap the expander for the final implant (usually silicone). In “one-step” implant operations, the final implant is placed at the time of mastectomy. Newly FDA-approved anatomic implants are teardrop shaped, appear more natural than the traditional round ones, and ripple less. Allograft and synthetic sheets of material are sometimes used to create a more natural look, to make up for weak and thin body tissues, and to build an additional layer between the implant and skin.
- Autologous Flaps use your own skin, fat and sometimes muscle from the abdomen, back (latissimus), thigh (gracilis), or buttock to create a potentially more natural breast reconstruction than implants can achieve. Flaps, however, create scars at the donor site, potential weakness in the donor area, and involve a longer operation than implants, with longer hospital stays and recovery periods.
- Nipple reconstruction is an option for those whose nipples are removed at the time of mastectomy. Either the breast skin itself, or a graft of skin from elsewhere on the body, is used to create a projecting nipple (no sensation). Tattoos can be surprisingly realistic recreating a tinted areola (the pigmented circle around a nipple).
- Prophylactic Bilateral Salpingo-Oopherectomy (BSO)
- Prophylactic BSO reduces future ovarian cancer (primary peritoneal cancer) risk to 1-2%.
- What is the ideal age? 10 years younger than the youngest affected relative with ovarian cancer; or by age 40; or earlier per patient desire; or as soon as childbearing is finished. Why the 10 years advice? Scientific studies show that the doubling rate of a cancer cell is such that the first cell most likely mutated 5-8 years before the cancer could ever be detected and diagnosed. So, the 10 years is meant to stop cancer before it starts.
- Consider freezing eggs or embryos between ages 30-40. Eggs are not fertilized, and contain just the woman’s half of a future baby, whereas embryos contain both egg and sperm united. This option is ideal for the woman who wants to have a BSO, but for a number of possible reasons, is not ready for pregnancy. She can preserve viable eggs/embryos, undergo BSO, and implant an embryo into her intact uterus when ready for motherhood.
- Controversial to be sure, Preimplantation Genetic Diagnosis (PGD) allows a BRCA carrier the chance to notpass on his/her genetic mutation. PGD analyzes embryos (obtained by IVF techniques) for the BRCA mutation, allowing only those without the mutation to be implanted into the uterus. A word of caution to any couples who both carry a BRCA-2 mutation: there is a 25% of creating a child with two faulty BRCA-2 genes, called Fanconi anemia. These children live a life fraught with medical issues and early death.
- Prophylactic BSO can be performed laparoscopically. Since the majority of the cancer risk lies in the part of the fallopian tube away from the uterus and toward the ovary, it is considered safe to keep the uterus. The chances of incidentally finding cancer in prophylactic BSO tissues ranges from 2-26%.
- Taking hormone replacement therapy (HRT) after BSO does not increase breast cancer risk in BRCA1 or BRCA2 mutation carriers.
- Performing BSO prior to mastectomies reduces breast cancer risk up to 68%; however, studies show no reduction in breast cancer risk if BSO is done after age 50.
Conclusion
No single management strategy can be universally recommended to BRCA1 and BRCA2 mutation carriers. Decisions depend on the unique life circumstances of each woman: her personal experiences, her dreams and goals, her “essence”, values, and convictions.